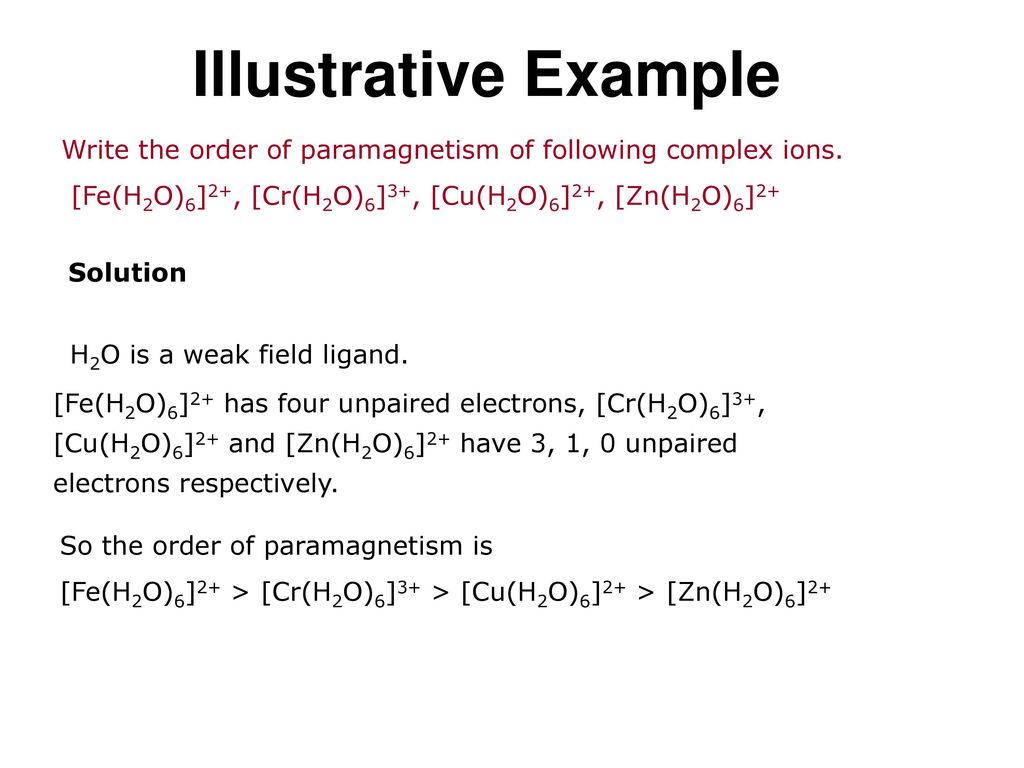
Identify The Geometry Of Fe H2o 6 3
For the complex FeH2O63 write the hybridisation magnetic character and spin of the complex. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds.
What Is The Hybridisation Of Fe Co 5 Quora
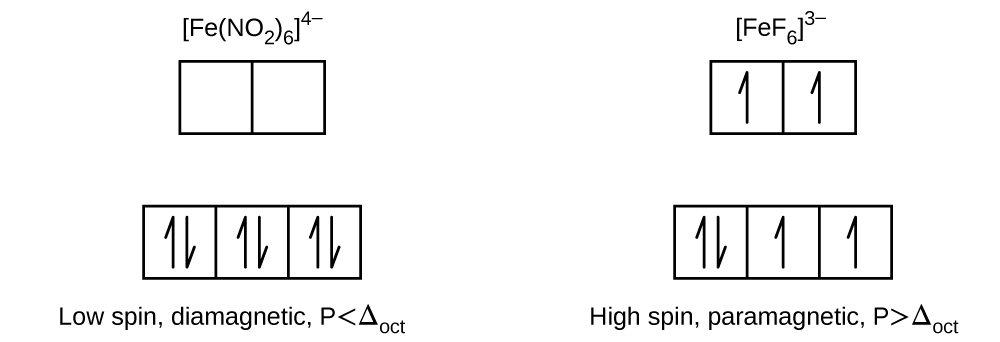
Draw the crystal field diagrams for FeNO 2 6 4 and FeF 6 3.

Identify the geometry of fe h2o 6 3. It often occurs as the trihydrate K 3 FeC 2 O 4 33H 2 O. As H2O is a weak field ligand no pairing of electrons takes place. What is the most likely molecular geometry of the compound K2FeNH3CN5.
The ions or molecules that bind to transition-metal ions to form these complexes are called ligands. This problem has been solved. Octahedral linear square planar trigonal planar tetrahedral.
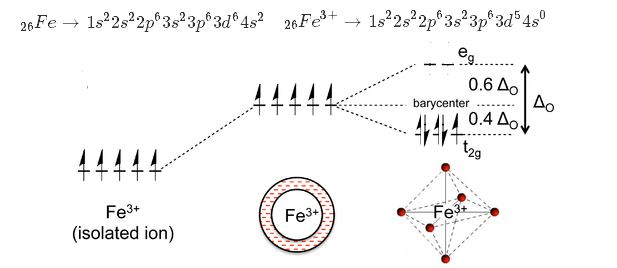
The electronic configuration of central metal ion Fe is. The oxidation number is synonymous with the oxidation state. Identify the geometry of Fe H2O63.
4 Fes 3 O2g 2 Fe2O3s Which of the following statements is correct. Therefore the number of unpaired electrons is 5. Site Preference in Spinels NiFe 2 O 4.
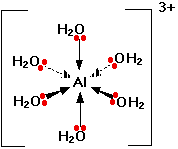
For example aqueous solutions of FeH 2O 6 3 are red CoH 2O 6 2 are pink NiH 2O 6 2 are green CuH 2O 6 2 are blue and ZnH 2O 6 2 are colorless. Because there are 6 ligands it has octahedral geometry. Question Bank Solutions 24397.
The atomic number of Fe is 26 and that of ion II ion is 24. Fe 3 O 4 CFCoFe 2 O 4 NiFe 2 O 4. Identify the geometry of Fe H2O6 3.
The central metal has oxidation number 3calculated as. The coordination number is 6. Thus the complex has octahedral geometry and is diamagentic because of the inner d orbital 3d is used in hybridisationthe complex C o N H 3 6 3 is called an inner orbital or low spin or spin paired complex The paramagnetic octahedral complex C o F 6 3 uses outer orbital 4d in hybridisation s p 3 d 2.
What geometry corresponds to a 3-coordinate complex. Tetrahedral geometry is not affected by this rule as it does not have a center of symmetry. As a consequence εfor tetrahedral complexes are 100 times more than the εfor octahedral comple escomplexes.
Thus it is strongly paramagnetic due to Presence of unpaired electrons. State whether each complex is high spin or low spin paramagnetic or diamagnetic and compare Δ oct to P for each complex. Upon pairing it has one unpaired electron.
Mostly predictable geometry. H C CH Bidentate. Both are crystalline compounds lime green in colour.
What is the nature of the t 2 g orbitals that are part of Δ o bonding. So the hybridization of the complex is d 2 sp 3. The green color of NiH 2O 6 2 turns blue when ammonia is added to give NiNH 36 2.
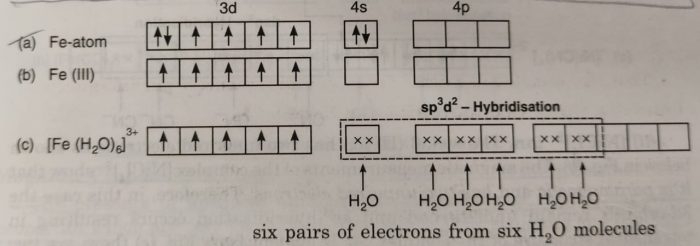
There are three bidentate ligands attached to the central metal 3 x 2 6. A For the complex Fe H2O63 write the hybridizationmagnetic character and spin of the complex. From Class 12 CBSE Previous Year Board Papers.
26 Therefore its electronic configuration is Ar 4s2 3d6 And that of Fe2 is 3d6 4s0. Chemistry questions and answers. B Fe is the limiting reactant and 175 mol of product can be produced.
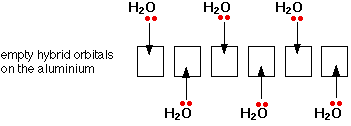
The coordination number is 4. The compound is a salt consisting of ferrioxalate anions FeC 2 O 4 3 3 and potassium cations K. To accomodate lone pairs from six H2O on.
In FeH2O62 central metal atom Fe has atomic no. Although the octahedral CoH 2O 6 2 are pink those of tetrahedral CoCl 4 2-are blue. Consider a complex FeCN 6 3.
It is thus called outer orbital or high spin or spin free complexThus. What is the geometry of the complex K2PtCl6. Feen 2Cl FeIII 6.
The number of atoms attached to the metal is coordination number of the metal. Coordination number 2 linear Coordination number 4 square planar or tetrahedral Coordination number 6 octahedral Ligands Molecules or anions rarely cations Molecules are given the molecular name but H2O is aqua and NH3 is ammine. A For the complex FeH 2 O 6 3 write the hybridizationmagnetic character and spin of the complexAtnumber.
Coordination compounds such as the FeCl 4-ion and CrCl 3 6 NH 3 are called such because they contain ions or molecules linked or coordinated to a transition metalThey are also known as complex ions or coordination complexes because they are Lewis acid-base complexes. The balanced chemical equation can be written as. Determining oxidation numbers from the Lewis structure Figure 1a is.
Anions are named with the anion name but with an ending of Œo. B Draw one of the geometrical isomers of the complex Pten 2 Cl 2 2 which is optically inactive. MCQ Online Tests 2.
3 2 2-3. Since H2O is a weak field kgand it cannot cause in pairing of electrons. Answer 1 of 5.
Fe 26 Ar 4s 2 3d 6. A Fe is the excess reactant and 175 mol of product can be produced. CN is a powerful field ligand and uses inner 3d-orbitals to form a low spin complex.
The complex Fe H 2 O 6 2 exhibits only σ-donor behavior. What is the coordination number of the Fe atom is K3FeC2O43. This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions ie.
B Draw one of the geometrical isomers of the complex Pt en2Cl22 which is optically inactive. Fe has 4s23d6configuration and therefore Fe3will be 3d5. Using ligand field theory draw the molecular orbital diagram mixing the d-orbitals on the metal and the molecular orbitals on the ligands and fill in the available electrons.
What is the coordination number of the Au atom in KAuCN2SCN2. Now in case of Fe H 2 O 6 2 ion the oxidation state of Fe atom is 2 and co-ordination number of central metal FeIIion ionis 6. 3 FeC 2 O 4 3.
FeH2O4OH2 How many geometric isomers of CoH2O4Cl2 are there. Fe H2O63 outer d- orbitals n d-orbitals are. CBSE CBSE Science Class 12.
Ligand Coordination IHgI -Ï NiCO 4 2 CoCN 4 3-CoNH 3 6 3 H H. Give the oxidation state of the metal number of d electrons and the number of unpaired electrons predicted for CoNH 3 6 Cl 3. Fe H 2 O Fe 3 O 4 H 2.
The same number of atoms of each element must exist on the reactant side and the product side of the equation. If 350 mol of Fe reacts with 300 mol of oxygen in the following reaction. The chemical reaction is given as.
Concept Notes Videos 725. The anion is a transition metal complex consisting of an iron atom in the 3 oxidation state and three. Since it has octahedral geometry as there are 6 ligands so its hybridization will be d2sp3.
Fe 3 4 Fe 2 C 2 N-3 6 3.
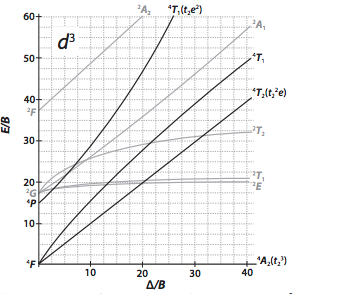
Using The Tanabe Sugano Diagrams In The Appendix Chegg Com
What Is The Hybridisation Of Mn Cn 6 3 Quora
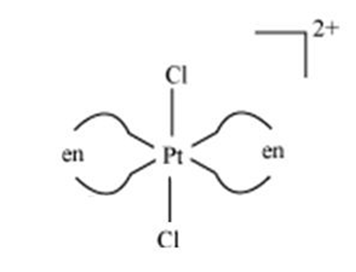
A For The Complex Fe H2o 6 3 Write The Hybridization Magnetic Character And Spin Of The Complex At Number Fe 26 B Draw One Of The Geometrical Isomers Of The Complex Pt En 2cl2 2 Which Is Optically Inactive From Chemistry
For The Complex Fe H2o 6 3 Write The Hybridization Magnetic Character And Spin Of The Complex Sarthaks Econnect Largest Online Education Community

Identify The Correct Order Of Wavelength Of Light Absorbed For The Following Complex Ions 1 Fe
For The Complex Ion Fe Cn 6 3 State I The Type Of Hybridisation Ii The Magnetic Behaviour Iii The Oxidation Number Of The Central Metal Atom
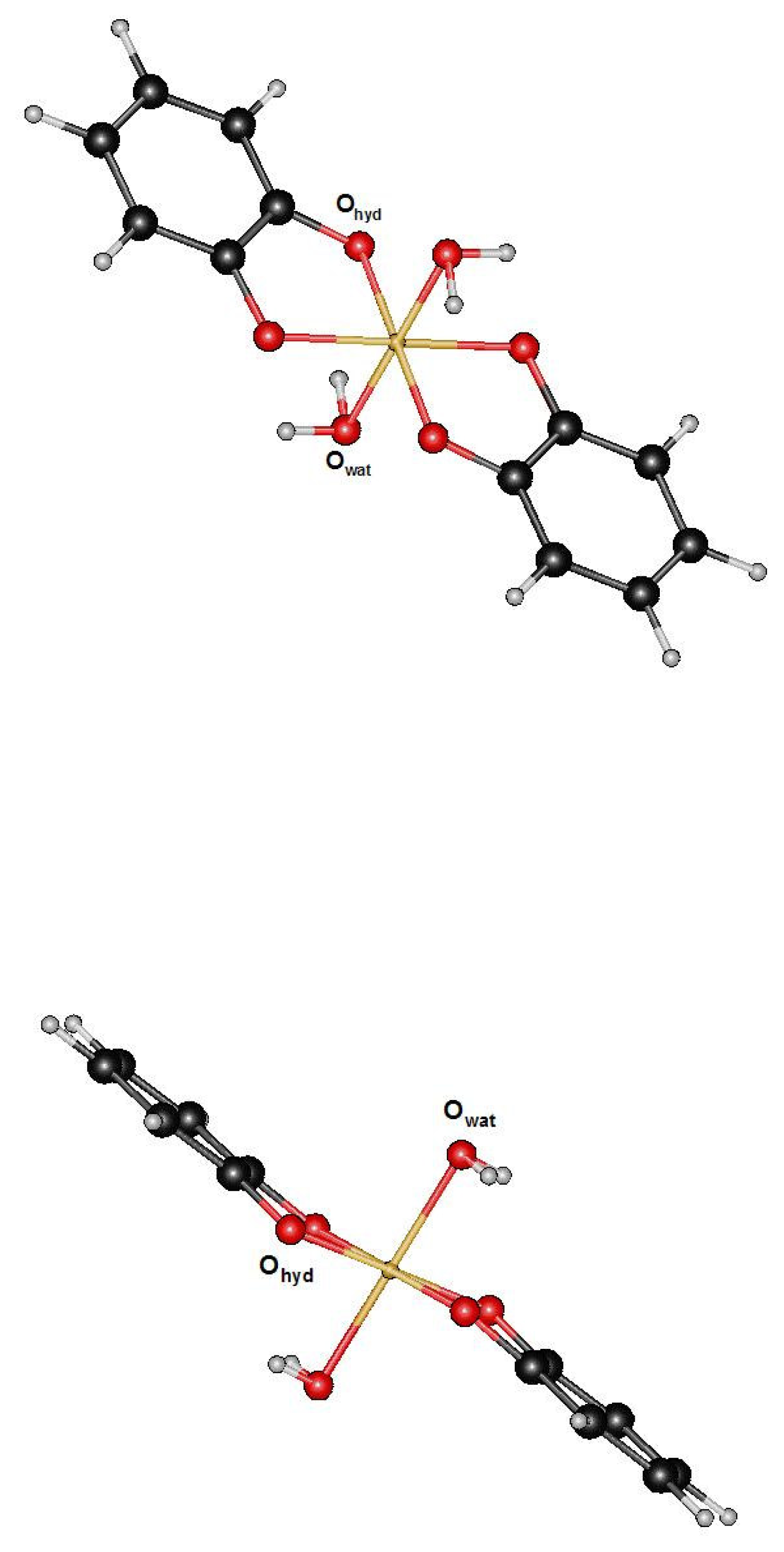
Ijms Free Full Text The Spasiba Force Field For Studying Iron Tannins Interactions Application To Fe3 Fe2 Catechol Complexe Html

Chemical Equations Conceptual Questions Teaching Resources Chemical Equation Equations Chemical
What Is The Hybridization Of Fe Cn 6 3 Quora

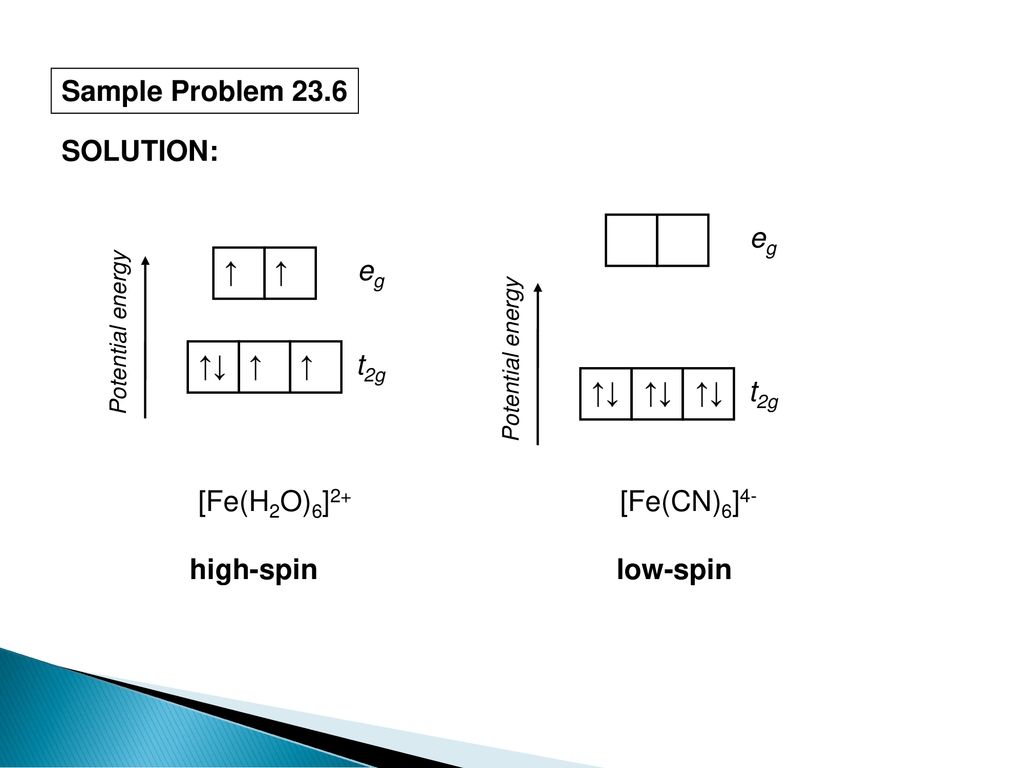
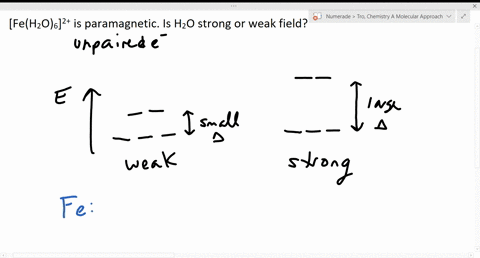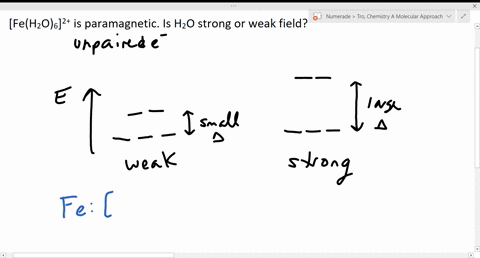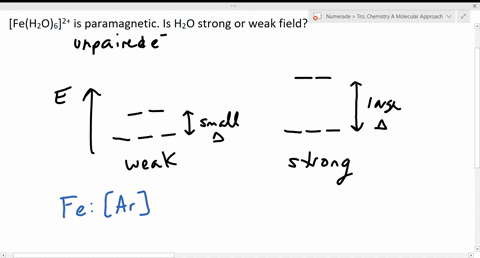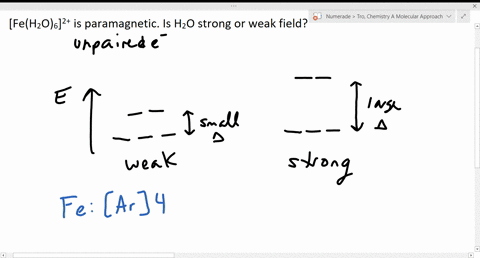
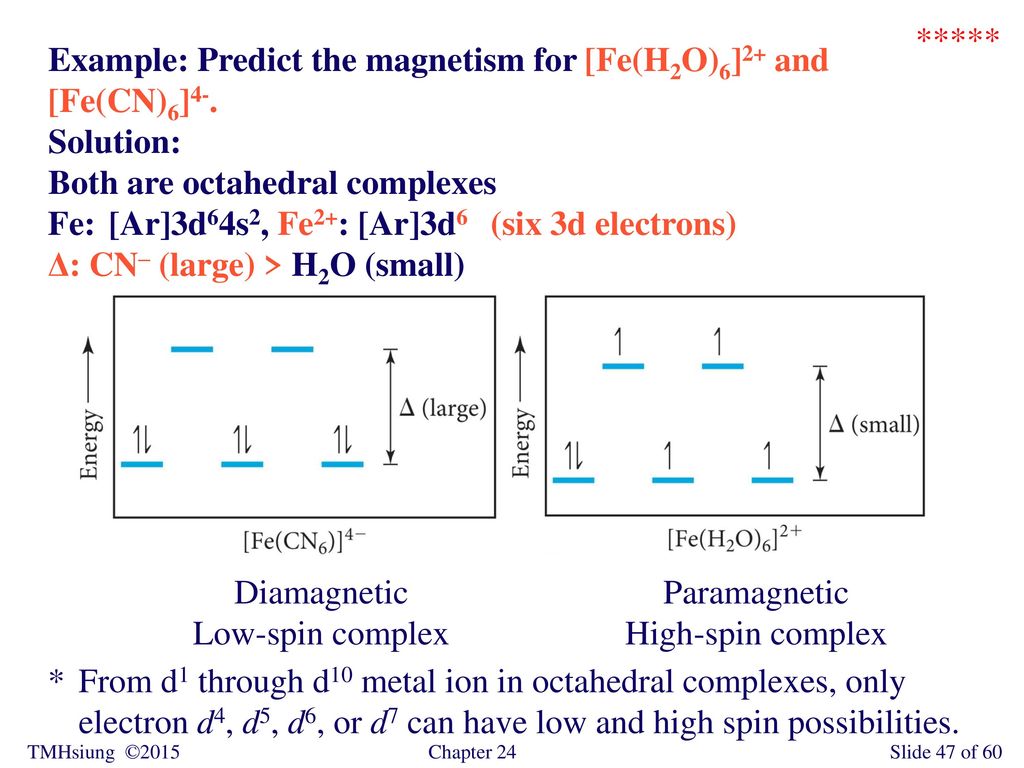
Solved The Complex Fe H2o 6 2 Is Paramagnetic Is The H2o Ligand Inducing A Strong Or Weak Field
19 3 Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry

Chemsolve Net Geometry And Magnetic Properties Of Fe H2o 6 2 Ion
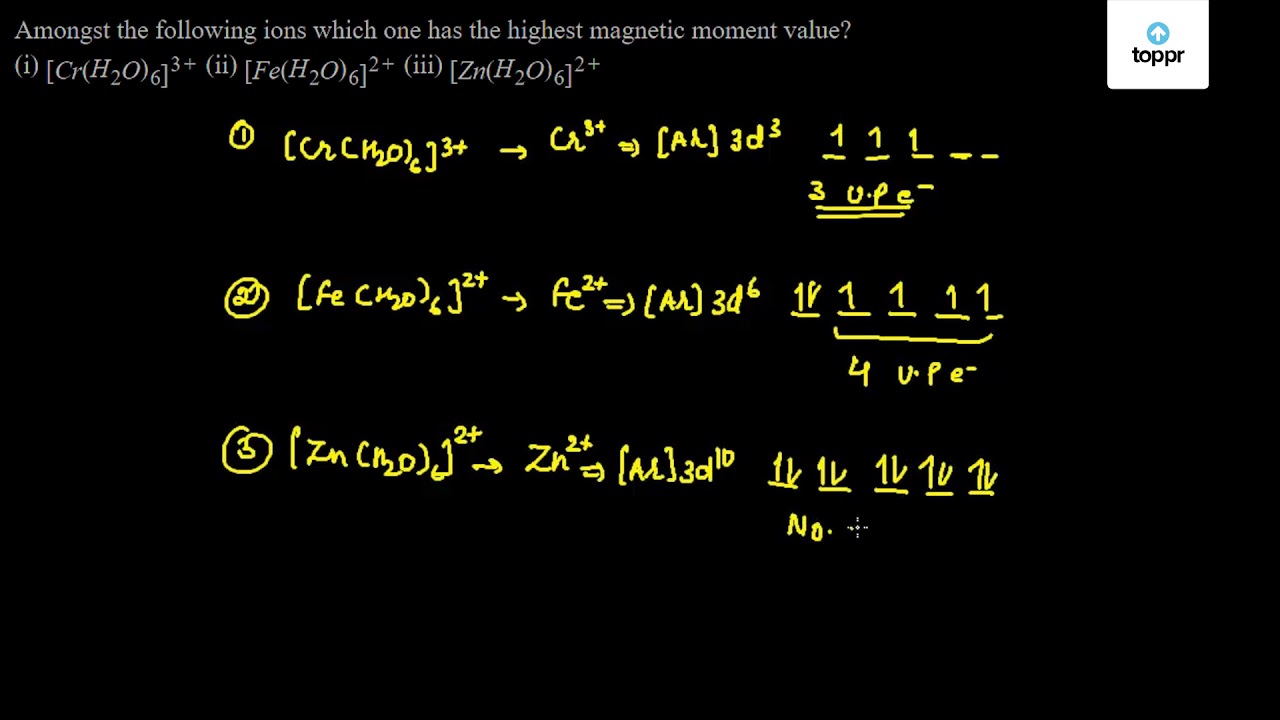
Amongst The Following Ions Which One Has The Highest Magnetic Moment Value I Cr H2o 6 3 Ii Fe H2o 6 2 Iii Zn H2o 6 2
Diamagnetic Paramagnetic Ni Co 4 Ni Cn 4 2 Nicl4 2 Vbt Hybridization Structure Iit Jee Neet Youtube
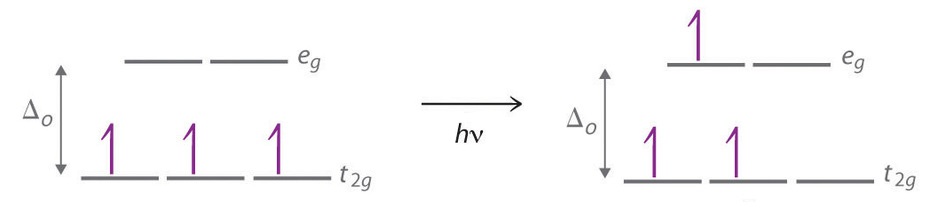
24 7 Color And The Colors Of Complexes Chemistry Libretexts

Do Increases In The Order Of Crcl6 3 Cr Cn 6 3 Cr C2o4 3 3

5f Transition Elements Diagram Quizlet
Cr H2o 6 3 Has A Much Higher Magnetic Property Than Mn Cn 6 4 Why Quora
Using The Tanabe Sugano Diagrams In The Appendix Chegg Com
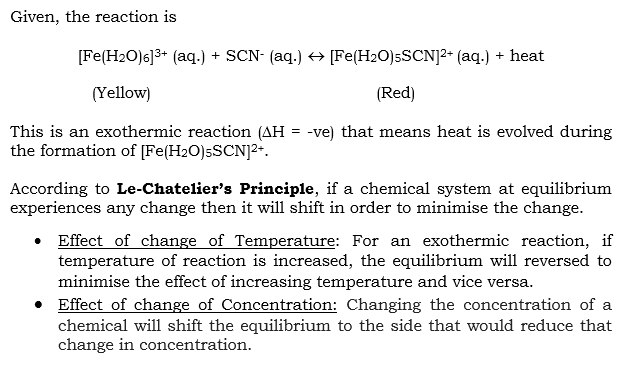
Answered Fe H2o 6 3 Aq Scn1 Bartleby

Using Crystal Field Theory Draw Energy Level Diagram Write Electronic Configuration Of The Central Metal Atom Ion And Determine The Magnetic Moment Value In The Following I Cof6 3 Co H2o 6 2 Co Cn 6 3
What Is The Hybridisation Of Co H2o 6 2 Quora
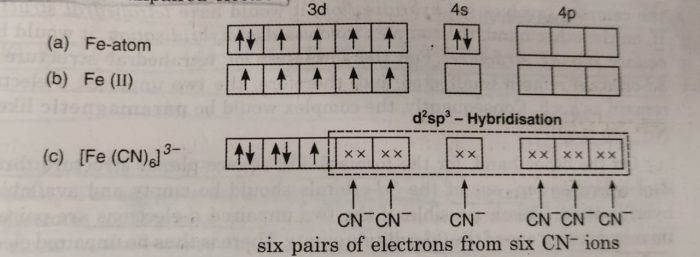
Valence Bond Theory For Bonding In Coordination Compounds Chemistry Class 12 Coordination Compounds
Coordination Compounds Ppt Video Online Download

Amongst The Following The Most Stable Complex Is A Fe H20 6 3 B Fe Nh3 6 3 C Fe C2o4 3 3 D Fec16

Among Fe H 2 O 6 3 Fe Cn 6 3 Fe Cl 6 3 Spe
What Is The Hybridization Of Fe Cn 6 3 Quora

Pdf Electronic Structure Of 3d M H2o 6 3 Ions From Sc Iii To Fe Iii A Quantum Mechanical Study Based On Dft Computations And Natural Bond Orbital Analyses
The Chemical Thesaurus Reaction Chemistry Database

Why Is Cr H2o 6 3 More Paramagnetic Than Fe Cn 6 4 Quora

Ch 23 Transition Elements And Their Coordination Compounds Ppt Download

1 Mn H2o 6 2 Draw The Possible Stereoisomers Give The Oxidation State Of The Metal Identify The Donor Homeworklib
Valence Bond Theory For Bonding In Coordination Compounds Chemistry Class 12 Coordination Compounds

3 2 5 Transition Metals Flashcards Quizlet
Metal Ligand Bonding In Transition Metal Complexes Ppt Download

Identify The Correct Order Of Wavelength Of Light Absorbed For The Following Complex Ions 1 Fe H20 3 Ii Fe Nh3 6 3 Iii Fe Cn 3 Iv Fef6 3 A I Iv Iii Ii B Iv I Iii Ii C Ii Iii I Iv D Iv I Ii Iii

Electron Configurations Of Transition Metal Atoms Ions Ppt Download
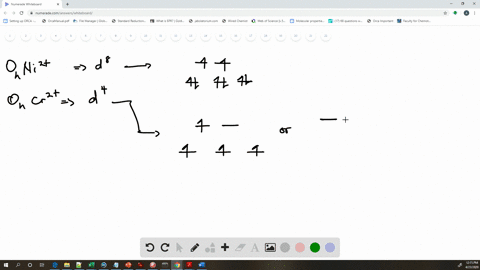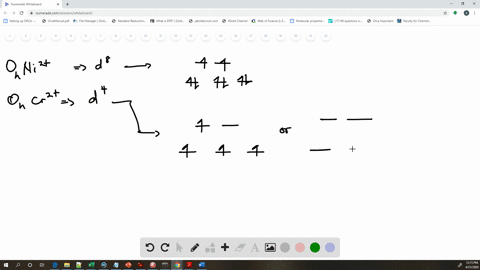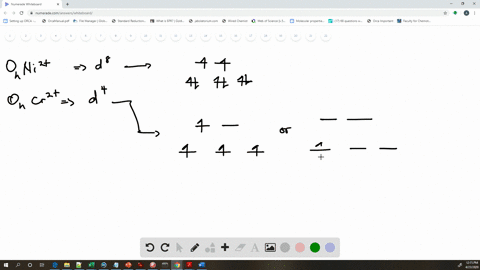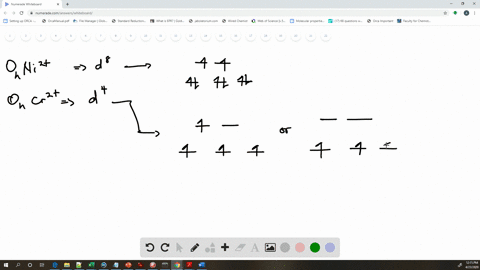
Solved The Complex Fe H2o 6 2 Is Paramagnetic Is The H2o Ligand Inducing A Strong Or Weak Field
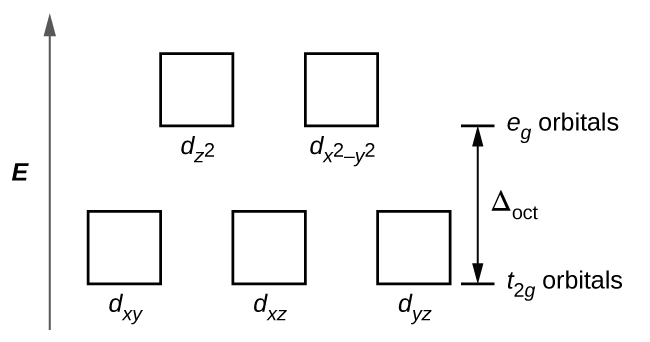
19 3 Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry

Pdf Electronic Structure Of 3d M H2o 6 3 Ions From Sc Iii To Fe Iii A Quantum Mechanical Study Based On Dft Computations And Natural Bond Orbital Analyses

Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As A2 Ib A Level Inorganic Chemistry Revision Notes

For The Complex Ion Fe Cn 6 3 State I The Type Of Hybridisation Ii The Magnetic Behaviour Iii The Oxidation Number Of The Central Metal Atom

10 The Complexes Mn H20 Fe H20 Mnclar And Fec14 All Have Magnetic Moments Of Nearly Homeworklib
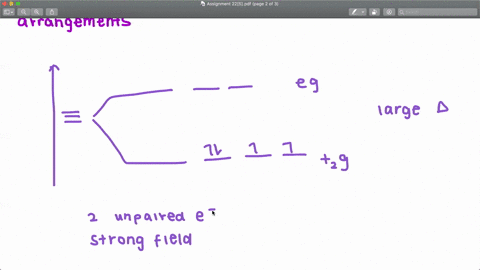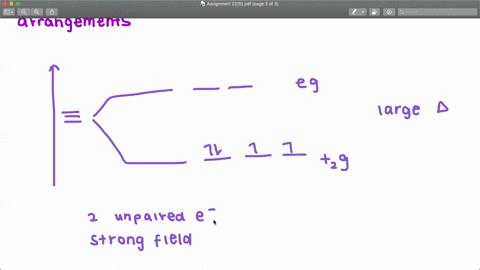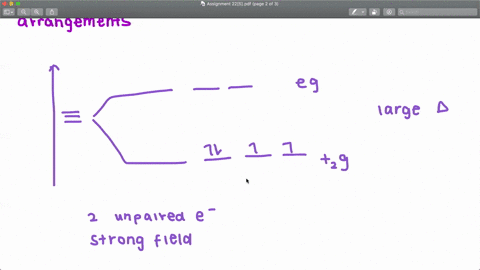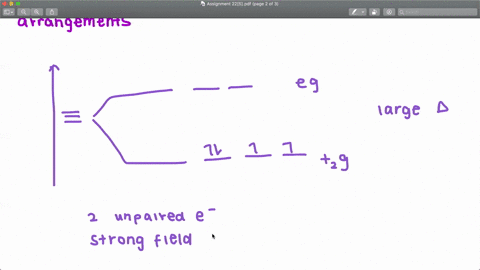
Solved The Complex Fe H2o 6 2 Is Paramagnetic Is The H2o Ligand Inducing A Strong Or Weak Field
Cr H2o 6 3 Has A Much Higher Magnetic Property Than Mn Cn 6 4 Why Quora
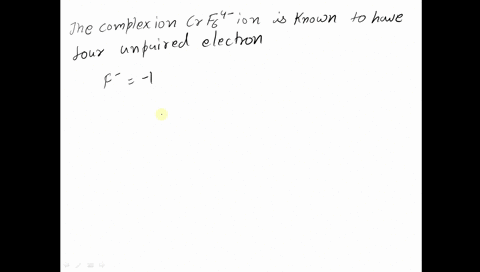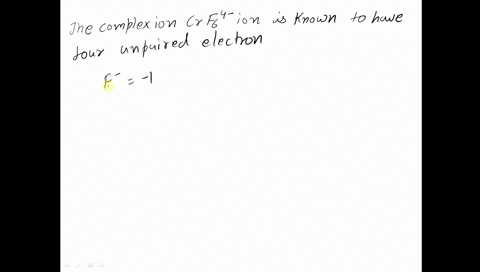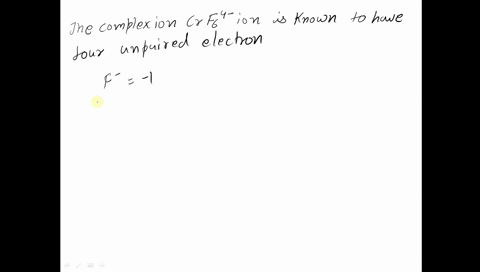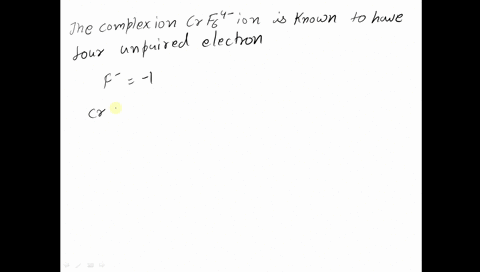
Solved The Complex Fe H2o 6 2 Is Paramagnetic Is The H2o Ligand Inducing A Strong Or Weak Field
Solved The Complex Fe H2o 6 2 Is Paramagnetic Is The H2o Ligand Inducing A Strong Or Weak Field
How Is Cr Nh3 6 3 Paramagnetic And Co Nh3 6 3 Is Diamagnetic Quora

Assertion A Fe Cn 6 3 Ion Shows Magnetic Moment Corresponding To Two Youtube

Jahn Teller Distortion Effect Theorem Examples Adichemistry
What Is The Hybridisation Of Co H2o 6 2 Quora

Chemsolve Net Geometry And Magnetic Properties Of Fe H2o 6 2 Ion
Fe Cn 6 4 Molecular Orbital Diagram

Fe Cn 6 4 And Fe H2o 6 2 Are Of Different Colours In Dilute Solutions Why

Lecture Note Chem 4236 Inorganic Chemistry By Dr Mrs Ppt Download
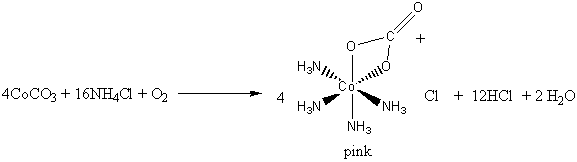
D Metal Complexes Practice Problems Answers
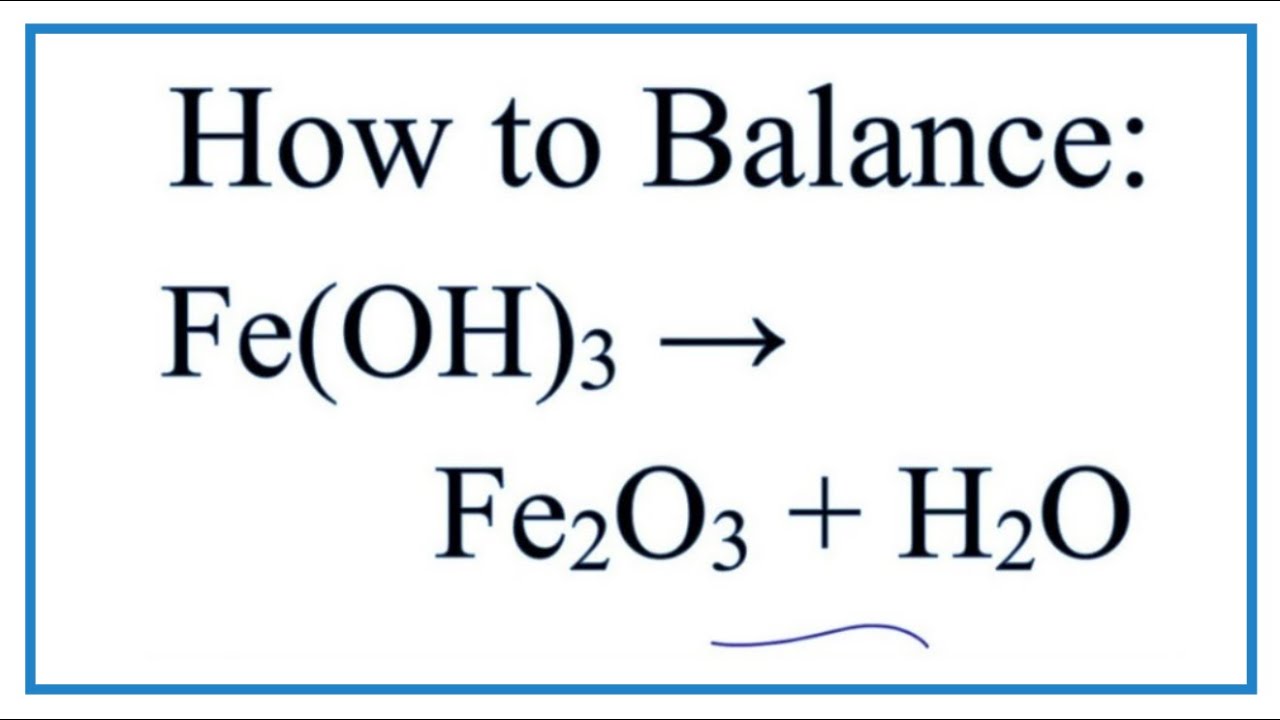
How To Balance Fe Oh 3 And Heat Fe2o3 H2o Decomposition Of Iron Iii Hydroxide Youtube
What Are The Crystal Field Splitting Energy And The Spin Only Moment In Bohr Magneton For The Complex K 3 Fe Cn 6 Socratic
What Is The Hybridisation Of Co H2o 6 2 Quora
Transition Metals And Coordination Compounds Ppt Download
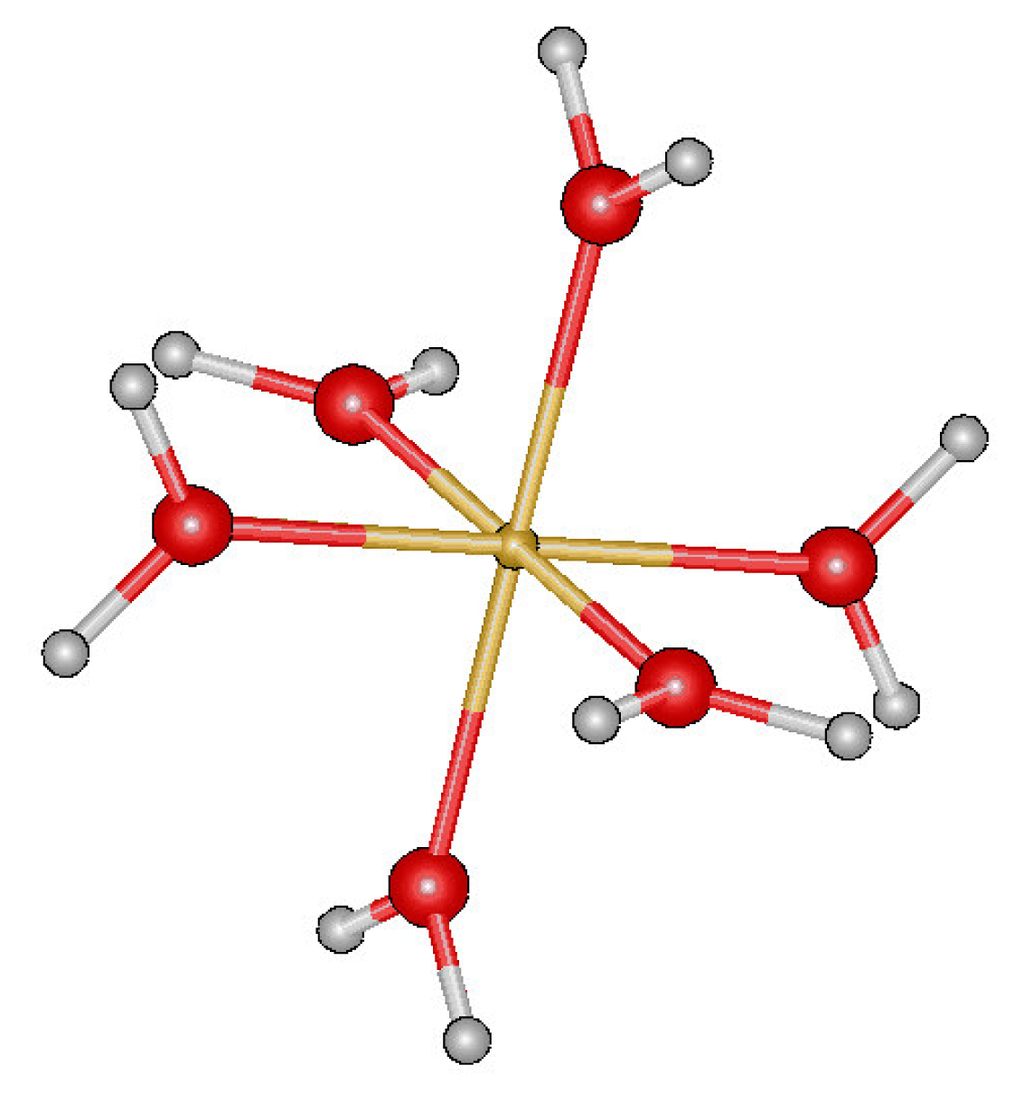
Ijms Free Full Text The Spasiba Force Field For Studying Iron Tannins Interactions Application To Fe3 Fe2 Catechol Complexe Html

Cft Stability Of Complex Fe H2o 6 3 High Spin Tn 12th Chemistry Unit 5 Coordination Chemistry Youtube

Pdf Electronic Structure Of 3d M H2o 6 3 Ions From Sc Iii To Fe Iii A Quantum Mechanical Study Based On Dft Computations And Natural Bond Orbital Analyses
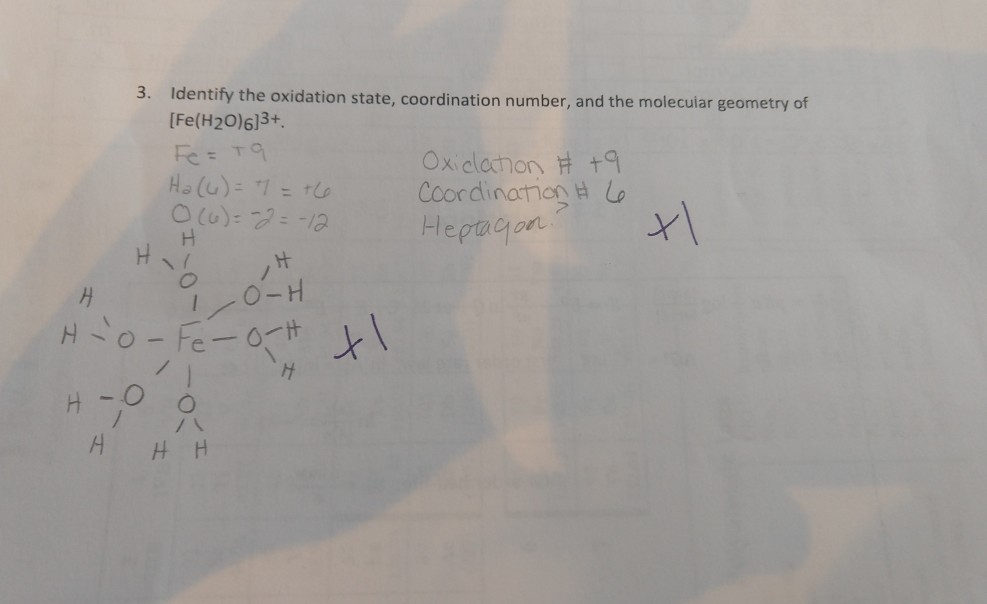
Solved 3 Identify The Oxidation State Coordination Number Chegg Com
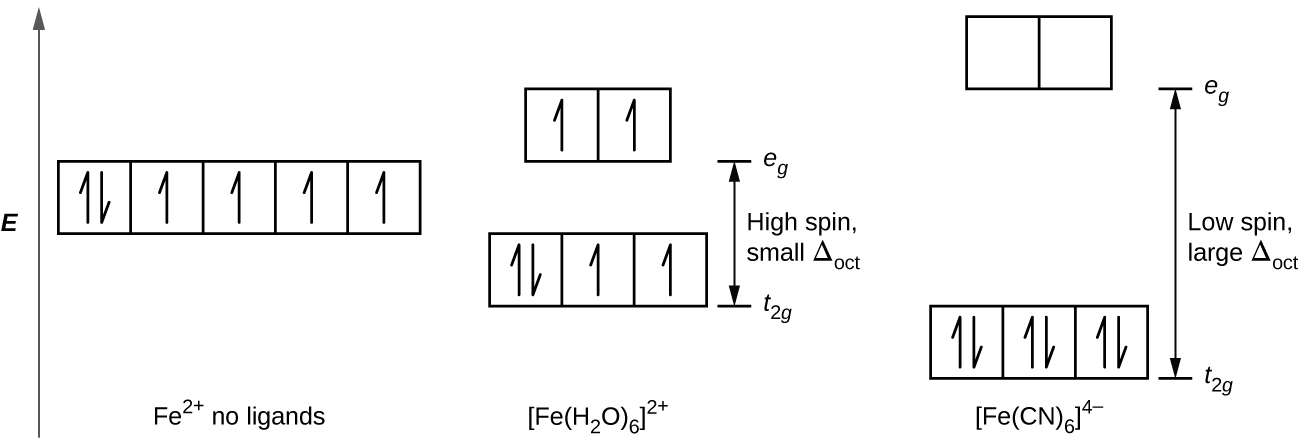
19 3 Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry

Fef 6 3 Has Fe Atom Hybridized With Unpaired Electrons Youtube
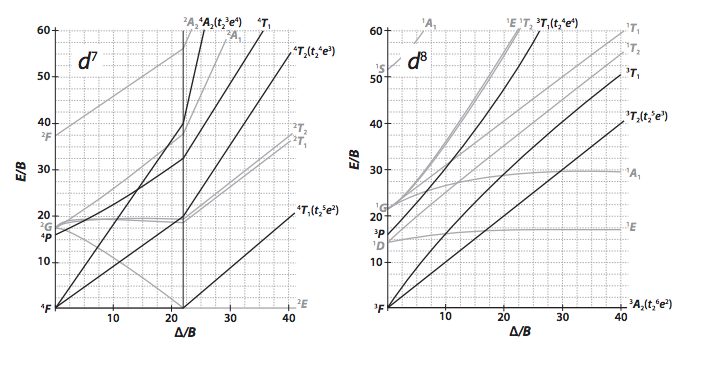
Using The Tanabe Sugano Diagrams In The Appendix Chegg Com

Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As A2 Ib A Level Inorganic Chemistry Revision Notes
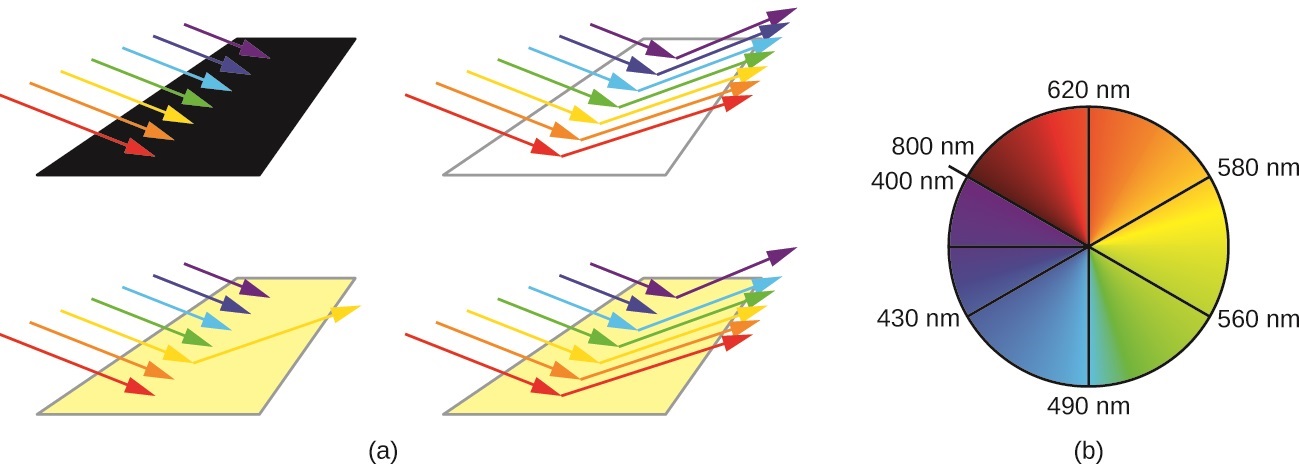
24 7 Color And The Colors Of Complexes Chemistry Libretexts
What Is The Name Of Fe H2o 6 So4 Quora
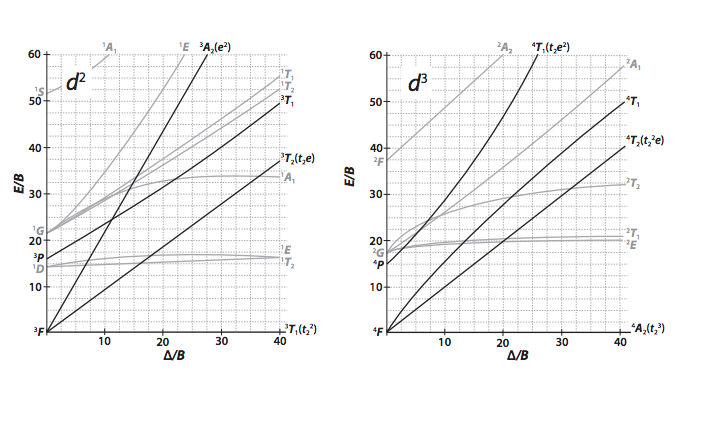
Using The Tanabe Sugano Diagrams In The Appendix Chegg Com
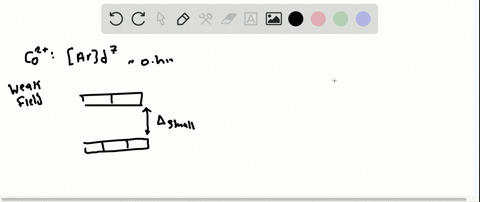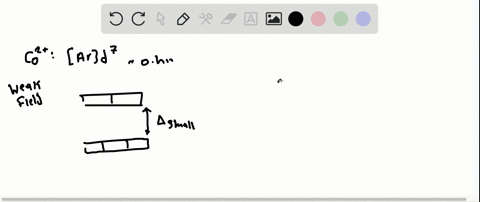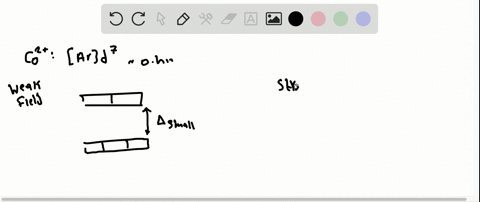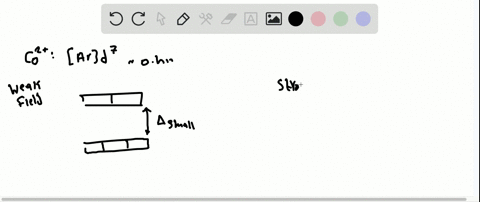
Solved The Complex Fe H2o 6 2 Is Paramagnetic Is The H2o Ligand Inducing A Strong Or Weak Field

Mechanistic Study Of Fe Iii Chelate Reduction In A Neutral Electro Fenton Process Sciencedirect
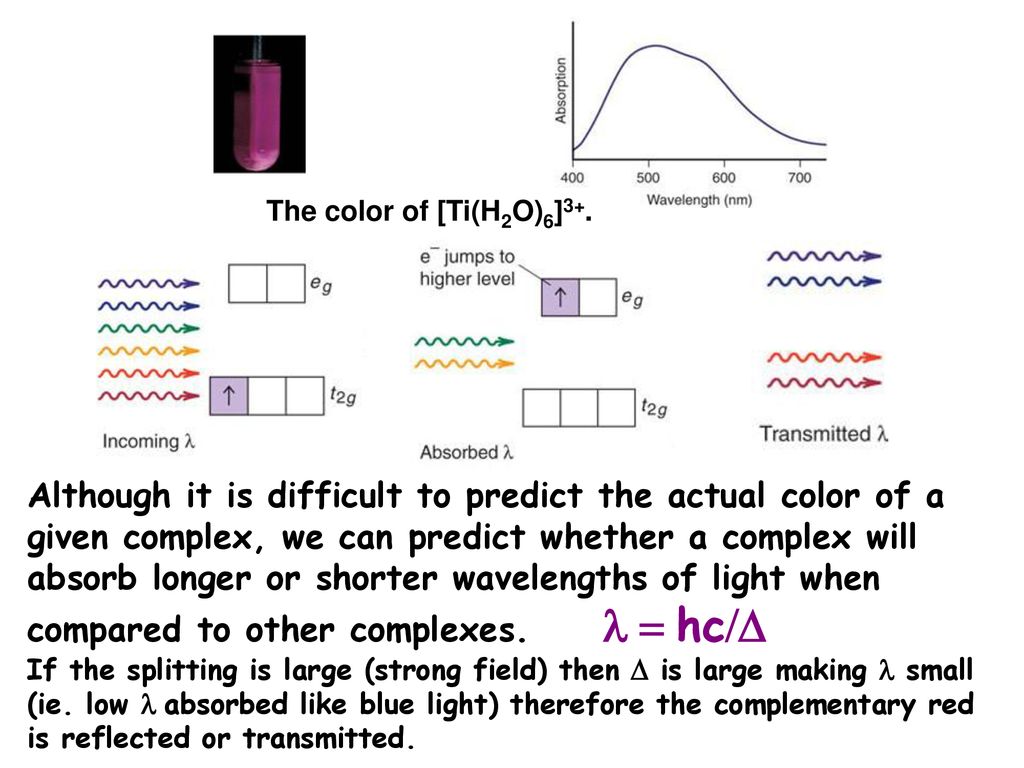
Transition Metal Chemistry Ppt Download
Solved The Mn Nh3 6 2 Ion Is Paramagnetic With Five Unpaired Electrons The Nh3 Ligand Is Usually A Strong Field Ligand Is Nh3 Acting As A Strong Field In This Case

Solved 2 The Following Are Low Spin Complexes Use Crystal Chegg Com

Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As A2 Ib A Level Inorganic Chemistry Revision Notes

What Is The Magnetic Character Of The Complex Fe H2o 6 3 Quora
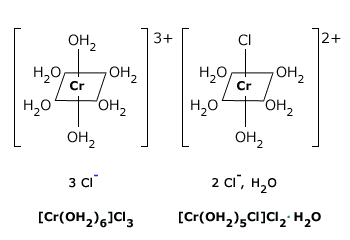
24 4 Isomerism In Coordination Complexes Chemistry Libretexts

3 2 6 Reactions Of Ions In Aqueous Solution Flashcards Quizlet

Answered The Characteristic Colour Of The 3 Bartleby
What Is Hybridisation Of Co H2o 6 3 Quora
Why Is K4 Fe Cn 6 Diamagnetic And Fe H2o 6 Cl2 Paramangnetic Quora